C O2 Co2 Precipitation Reaction
Precipitation Reactions
- Page ID
- 727
Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid chosen a precipitate. Whether or not such a reaction occurs tin can exist determined by using the solubility rules for common ionic solids. Because non all aqueous reactions course precipitates, one must consult the solubility rules earlier determining the state of the products and writing a net ionic equation. The power to predict these reactions allows scientists to determine which ions are present in a solution, and allows industries to form chemicals by extracting components from these reactions.
Properties of Precipitates
Precipitates are insoluble ionic solid products of a reaction, formed when certain cations and anions combine in an aqueous solution. The determining factors of the formation of a precipitate tin can vary. Some reactions depend on temperature, such as solutions used for buffers, whereas others are dependent simply on solution concentration. The solids produced in precipitate reactions are crystalline solids, and can exist suspended throughout the liquid or fall to the bottom of the solution. The remaining fluid is called supernatant liquid. The two components of the mixture (precipitate and supernate) can exist separated by various methods, such equally filtration, centrifuging, or decanting.
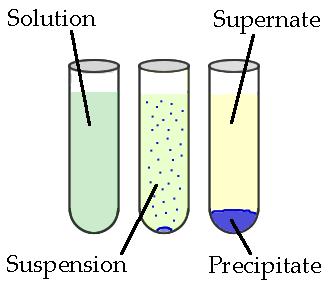
Precipitation and Double Replacement Reactions
The use of solubility rules crave an understanding of the way that ions react. Most precipitation reactions are single replacement reactions or double replacement reactions. A double replacement reaction occurs when two ionic reactants dissociate and bond with the respective anion or cation from the other reactant. The ions replace each other based on their charges equally either a cation or an anion. This can exist thought of as "switching partners"; that is, the ii reactants each "lose" their partner and grade a bond with a different partner:

A double replacement reaction is specifically classified as a precipitation reaction when the chemical equation in question occurs in aqueous solution and i of the of the products formed is insoluble. An example of a precipitation reaction is given below:
\[CdSO_{4(aq)} + K_2S_{(aq)} \rightarrow CdS_{(due south)} + K_2SO_{4(aq)}\]
Both reactants are aqueous and i product is solid. Because the reactants are ionic and aqueous, they dissociate and are therefore soluble. All the same, there are six solubility guidelines used to predict which molecules are insoluble in water. These molecules form a solid precipitate in solution.
Solubility Rules
Whether or not a reaction forms a precipitate is dictated by the solubility rules. These rules provide guidelines that tell which ions form solids and which remain in their ionic form in aqueous solution. The rules are to exist followed from the top downwardly, pregnant that if something is insoluble (or soluble) due to rule ane, it has precedence over a higher-numbered rule.
- Salts formed with group one cations and \(NH_4^+\) cations are soluble. There are some exceptions for sure \(Li^+\) salts.
- Acetates (\(C_2H_3O_2^-\)), nitrates (\(NO_3^-\)), and perchlorates (\(ClO_4^-\)) are soluble.
- Bromides, chlorides, and iodides are soluble.
- Sulfates (\(SO_4^{ii-}\)) are soluble with the exception of sulfates formed with \(Ca^{two+}\), \(Sr^{2+}\), and \(Ba^{2+}\).
- Salts containing argent, lead, and mercury (I) are insoluble.
- Carbonates (\(CO_3^{ii-}\)), phosphates (\(PO_4^{three-}\)), sulfides, oxides, and hydroxides (\(OH^-\)) are insoluble. Sulfides formed with group two cations and hydroxides formed with calcium, strontium, and barium are exceptions.
If the rules land that an ion is soluble, then it remains in its aqueous ion form. If an ion is insoluble based on the solubility rules, then it forms a solid with an ion from the other reactant. If all the ions in a reaction are shown to exist soluble, then no precipitation reaction occurs.
Net Ionic Equations
To understand the definition of a net ionic equation, recall the equation for the double replacement reaction. Because this particular reaction is a precipitation reaction, states of matter tin be assigned to each variable pair:
A B( aq ) + C D( aq ) → A D( aq ) + C B( s )
The first stride to writing a net ionic equation is to split up the soluble (aqueous) reactants and products into their respective cations and anions. Precipitates do not dissociate in water, so the solid should not be separated. The resulting equation looks like that below:
A+ ( aq ) + B- ( aq ) + C+ ( aq ) + D - ( aq ) → A+ ( aq ) + D - ( aq ) + C B( s )
In the equation above, A+ and D - ions are nowadays on both sides of the equation. These are chosen spectator ions because they remain unchanged throughout the reaction. Since they go through the equation unchanged, they can be eliminated to show the net ionic equation:
C+ (aq) + B- (aq) → C B (s)
The net ionic equation simply shows the precipitation reaction. A net ionic equation must exist balanced on both sides not but in terms of atoms of elements, simply besides in terms of electric charge. Precipitation reactions are usually represented solely past internet ionic equations. If all products are aqueous, a internet ionic equation cannot exist written considering all ions are canceled out as spectator ions. Therefore, no precipitation reaction occurs.
Applications and Examples
Precipitation reactions are useful in determining whether a certain element is present in a solution. If a precipitate is formed when a chemical reacts with lead, for example, the presence of pb in water sources could be tested by calculation the chemical and monitoring for precipitate germination. In addition, atmospheric precipitation reactions tin can be used to extract elements, such equally magnesium from seawater. Precipitation reactions even occur in the human body betwixt antibodies and antigens; however, the surroundings in which this occurs is still beingness studied.
Example 1
Complete the double replacement reaction and so reduce it to the cyberspace ionic equation.
\[NaOH_{(aq)} + MgCl_{2 \;(aq)} \rightarrow \]
First, predict the products of this reaction using knowledge of double replacement reactions (remember the cations and anions "switch partners").
\[2NaOH_{(aq)} + MgCl_{2\;(aq)} \rightarrow 2NaCl + Mg(OH)_2\]
Second, consult the solubility rules to make up one's mind if the products are soluble. Group 1 cations (\(Na^+\)) and chlorides are soluble from rules 1 and 3 respectively, and so \(NaCl\) will be soluble in water. However, rule 6 states that hydroxides are insoluble, and thus \(Mg(OH)_2\) will grade a precipitate. The resulting equation is the following:
\[2NaOH(aq) + MgCl_{2\;(aq)} \rightarrow 2NaCl_{(aq)} + Mg(OH)_{2\;(southward)}\]
Third, separate the reactants into their ionic forms, equally they would exist in an aqueous solution. Be sure to residual both the electric charge and the number of atoms:
\[2Na^+_{(aq)} + 2OH^-_{(aq)} + Mg^{2+}_{(aq)} + 2Cl^-_{(aq)} \rightarrow Mg(OH)_{2\;(s)} + 2Na^+_{(aq)} + 2Cl^-_{(aq)}\]
Lastly, eliminate the spectator ions (the ions that occur on both sides of the equation unchanged). In this instance, they are the sodium and chlorine ions. The final net ionic equation is:
\[Mg^{2+}_{(aq)} + 2OH^-_{(aq)} \rightarrow Mg(OH)_{2(s)}\]
Example ii
Complete the double replacement reaction and so reduce it to the net ionic equation.
\[CoCl_{two\;(aq)} + Na_2SO_{4\;(aq)} \rightarrow\]
Solution
The predicted products of this reaction are \(CoSO_4\) and \(NaCl\). From the solubility rules, \(CoSO_4\) is soluble considering rule 4 states that sulfates (\(SO_4^{two-}\)) are soluble. Similarly, we find that \(NaCl\) is soluble based on rules i and 3. After balancing, the resulting equation is as follows:
\[CoCl_{2\;(aq)} + Na_2SO_{4\;(aq)} \rightarrow CoSO_{4\;(aq)} + 2 NaCl_{(aq)}\]
Carve up the species into their ionic forms, as they would exist in an aqueous solution. Balance the charge and the atoms. Cancel out all spectator ions (those that appear as ions on both sides of the equation.):
Co two - (aq) + 2Cl -(aq) + 2Na + (aq) + SOfour ii -(aq) → Co 2 - (aq) + SO4 ii -(aq) + 2Na + (aq) + 2Cl -(aq)
No atmospheric precipitation reaction
This item case is important because all of the reactants and the products are aqueous, pregnant they cancel out of the net ionic equation. There is no solid precipitate formed; therefore, no precipitation reaction occurs.
Do Problems
Write the internet ionic equation for the potentially double displacement reactions. Make certain to include the states of affair and residuum the equations.
- \(Atomic number 26(NO_3)_{iii\;(aq)} + NaOH_{(aq)} \rightarrow\)
- \(Al_2(SO_4)_{three\;(aq)} + BaCl_{2\;(aq)} \rightarrow\)
- \(HI_{(aq)} + Zn(NO_3)_{2\;(aq)} \rightarrow\)
- \(CaCl_{2\;(aq)} + Na_3PO_{four\;(aq)} \rightarrow\)
- \(Pb(NO_3)_{ii\;(aq)} + K_2SO_{4 \;(aq)} \rightarrow\)
Solutions
1. Regardless of physical country, the products of this reaction are \(Fe(OH)_3\) and \(NaNO_3\). The solubility rules predict that \(NaNO_3\) is soluble because all nitrates are soluble (rule ii). Nonetheless, \(Fe(OH)_3\) is insoluble, considering hydroxides are insoluble (rule vi) and \(Fe\) is not 1 of the cations which results in an exception. Later on dissociation, the ionic equation is as follows:
\[Fe^{3+}_{(aq)} + NO^-_{3\;(aq)} + Na^+_{(aq)} + 3OH^-_{(aq)} \rightarrow Fe(OH)_{iii\;(s)} + Na^+_{(aq)} + NO^-_{3\;(aq)}\]
Canceling out spectator ions leaves the internet ionic equation:
\[Fe^{3+}_{(aq)} + OH^-_{(aq)} \rightarrow Fe(OH)_{\;3(south)}\]
2. From the double replacement reaction, the products are \(AlCl_3\) and \(BaSO_4\). \(AlCl_3\) is soluble because it contains a chloride (rule 3); however, \(BaSO_4\) is insoluble: it contains a sulfate, but the \(Ba^{2+}\) ion causes it to be insoluble considering it is one of the cations that causes an exception to rule iv. The ionic equation is (after balancing):
\[2Al^{three+}_{(aq)} + 6Cl^-_{(aq)} + 3Ba^{ii+}_{(aq)} + 3SO^{ii-}_{four\;(aq)} \rightarrow 2 Al^{3+}_{(aq)} +6Cl^-_{(aq)} + 3BaSO_{4\;(s)}\]
Canceling out spectator ions leaves the following net ionic equation:
\[Ba^{2+}_{(aq)} + Then^{2-}_{four\;(aq)} \rightarrow BaSO_{four\;(s)}\]
iii. From the double replacement reaction, the products \(HNO_3\) and \(ZnI_2\) are formed. Looking at the solubility rules, \(HNO_3\) is soluble because information technology contains nitrate (dominion two), and \(ZnI_2\) is soluble because iodides are soluble (rule three). This means that both the products are aqueous (i.e. dissociate in h2o), and thus no precipitation reaction occurs.
4. The products of this double replacement reaction are \(Ca_3(PO_4)_2\) and \(NaCl\). Rule one states that \(NaCl\) is soluble, and co-ordinate to solubility rule 6, \(Ca_3(PO_4)_2\) is insoluble. The ionic equation is:
\[Ca^{ii+}_{(aq)}+ Cl^-_{(aq)} + Na^+_{(aq)} + PO^{three-}_{four\;(aq)} \rightarrow Ca_3(PO_4)_{ii\;(s)} + Na^+_{(aq)} + Cl^-_{(aq)}\]
Later on canceling out spectator ions, the net ionic equation is given below:
\[Ca^{2+}_{(aq)} + PO^{three-}_{4\;(aq)} \rightarrow Ca_3(PO_4)_{2\;(south)}\]
5. The first product of this reaction, \(PbSO_4\), is soluble according to rule four because information technology is a sulfate. The second product, \(KNO_3\), is also soluble because it contains nitrate (dominion 2). Therefore, no precipitation reaction occurs.
References
- Campbell, Dan, Linus Pauling, and Davis Pressman. "The Nature of the Forces Between Antigen and Antibody and of the Precipitation Reaction." Physiological Reviews 23.3 (1943): 203-219. Online.
- Harwood, William, F Herring, Jeffry Madura, and Ralph Petrucci. General Chemical science. 9th ed. Upper Saddle River: Pearson Pretence Hall, 2007. Print.
- Freeouf, J.L, Grischkowsky, D., McInturff, D.T., Warren, A.C., & Woodall, J.G. (1990). Arsenic precipitates and the semi-insulating properties of gaas buffer layers grown by low-temperature molecular beam epitaxy. Practical Physics Messages, 57(13)
- Petrucci, et al. General Chemistry: Principles & Modern Applications. 9th ed. Upper Saddle River, New Jersey 2007.
Contributors and Attributions
- Julie Schaffer (UCD), Corinne Herman (UCD)
C O2 Co2 Precipitation Reaction,
Source: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Main_Group_Reactions/Reactions_in_Aqueous_Solutions/Precipitation_Reactions
Posted by: larkinusand2001.blogspot.com


0 Response to "C O2 Co2 Precipitation Reaction"
Post a Comment